
For example, sodium has one valence electron, in its outermost shell, so in ionized form it is commonly found with one lost electron, as Na +.
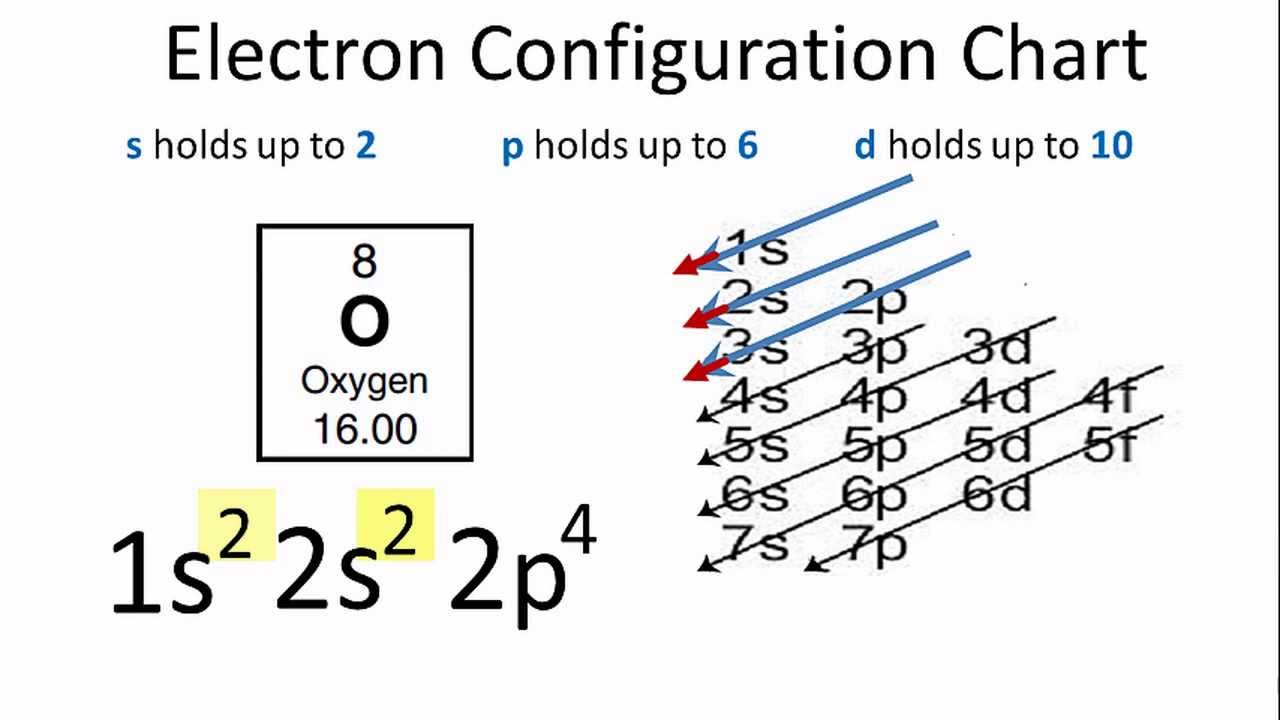
#Oxygen charge of ion full#
For this reason, ions tend to form in ways that leave them with full orbital blocks. Particularly great increases occur after any given block of atomic orbitals is exhausted of electrons. The nth ionization energy of an atom is the energy required to detach its nth electron after the first n − 1 electrons have already been detached.Įach successive ionization energy is markedly greater than the last. The energy required to detach an electron in its lowest energy state from an atom or molecule of a gas with less net electric charge is called the ionization potential, or ionization energy. + is not stable because of an incomplete valence shell around nitrogen and is in fact a radical ion.The distinction between this and the removal of an electron from the whole molecule is important in large systems because it usually results in much more stable ions with complete electron shells.
The charge has been added by the addition of a proton (H +) not the addition or removal of electrons. Ammonia and ammonium have the same number of electrons in essentially the same electronic configuration but differ in protons. A simple example of this is the ammonium ion NH 4 + which can be formed by ammonia NH 3 accepting a proton, H +. Polyatomic and molecular ions are often formed by the combination of elemental ions such as H + with neutral molecules or by the gain of such elemental ions from neutral molecules.
An electrostatic potential map of the nitrate ion ( N O 3 −).


 0 kommentar(er)
0 kommentar(er)
